Engineers turn dirty water into clean hydrogen
New Princeton research shows that reclaimed wastewater can replace purified water in hydrogen production, cutting treatment costs by 47%.
By Anthropocene Team October 30, 2025
When made by splitting water using renewable electricity, hydrogen promises to be a green fuel for transportation and industries. The process requires large amounts of clean fresh water, though, which stresses local water supplies and adds a cost.
made by splitting water using renewable electricity, hydrogen promises to be a green fuel
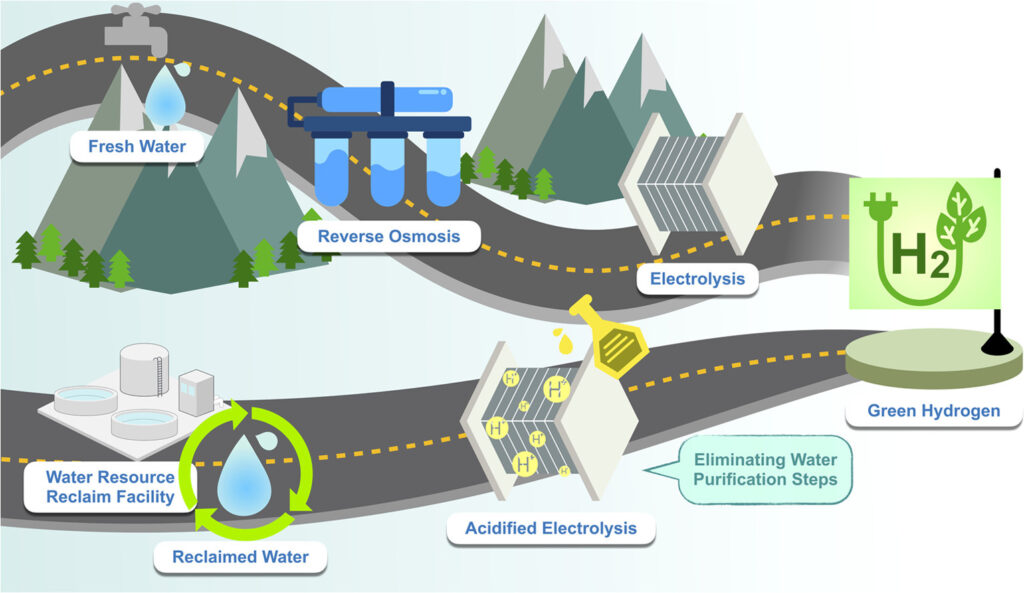
New research shows that it is possible to use reclaimed wastewater as a source for hydrogen. This could reduce the cost of treating water by 47%, in turn cutting the cost of producing green hydrogen from water, a team from Princeton University reports in the journal Water Research.
“Hydrogen infrastructure generally competes with local fresh water use,” said Z. Jason Ren, a professor of civil and environmental engineering. “But every town has a wastewater treatment plant, and that’s a very distributed source of water for the hydrogen economy.”
“every town has a wastewater treatment plant, and that’s a very distributed source of water for the hydrogen economy.”
Electrolysis involves passing electric current through water using metal electrodes to free hydrogen and oxygen. A catalyst if often added to speed up the reaction. The water typically needs to be ultra pure, otherwise impurities can damage the specialized membranes within the electrolyzer.
The cost of purifying water is small in the overall scheme of producing hydrogen by electrolysis, Ren and his team write in the paper. But sustainable water sourcing remains a critical bottleneck. This is why researchers have been exploring alternative supplies, such as reclaimed water, seawater, or saline water. Using these water sources will be “essential to scale the H2 economy without competing for precious freshwater resources,” they say.
A clever rooftop panel could make hydrogen fuel cheap—and commonplace
Producing hydrogen from seawater is challenging because the catalyst usually doesn’t work as efficiently as it does in pure water, salty water corrodes electrodes, and chlorine in the water can lead to side reactions.
The Princeton team used reclaimed wastewater. This is treated water from wastewater treatment plants that is suitable for discharge into the environment or for non-drinking uses like irrigation. Just like with seawater though, various water impurities in reclaimed water impacts the system performance and longevity.
So the researchers ran experiments in the laboratory to see exactly how contaminants affect the electrolyzer. They found that certain ions, particularly calcium and magnesium, significantly impacted the electrolysis process by clogging the electrolyzer membrane.
To overcome the issue, the team acidified the water with sulfuric acid. Protons from the acid essentially dislodge the ion impurities in the membrane and enhance ion transport, sustaining electrical current and enabling continuous hydrogen production. This simple solution extended the electrolyzer’s operational lifetime from under 8 hours to more than 300 hours.
According to the researchers’ calculations, using reclaimed wastewater rather than purified water could reduce the cost of treating water for hydrogen production by about 47% and the energy cost by about 62%.
“This work highlights the practical feasibility of reclaimed water electrolysis, enabling wastewater facilities to contribute to the broader integration of clean energy transition,” the team writes.
Source: Lin Du et al, Electrolytic hydrogen production from acidified wastewater effluent. Water Research, 2026
Highlights
- •Identified primary degradation mechanism in reclaimed water electrolysis.
- •Diagnosed membrane cation fouling and recovered with acidification treatment.
- •Maintains H2 Faradaic efficiency above 99 % with acidified reclaimed water feed.
- •Acid step cuts water treatment cost by 67.2 % and energy intensity by 62.9 %.
Abstract
Clean hydrogen provides a pathway to a sustainable energy future. However, current commercial water electrolyzers rely on high purity water, which can add significant stress on local water supply and additional economic burdens. To lessen the reliance on freshwater in electrolytic hydrogen production, alternative water sources such as reclaimed water (RW) from wastewater treatment facilities could be used. However, little is known how various water impurities in such source water impact the system performance and longevity. In this study, we characterized such impacts in lab-scale water electrolysis systems, and the results revealed that RW impurities primarily affected the membrane through contamination by cations. By acidifying the RW feedstock, we successfully extended electrolyzer stability from 8 h to over 300 h at a current density of 150 mA cm-2. Furthermore, this acidification step reduced cost by 46.9 % and energy intensity by 61.8 % via the elimination of multi-stage RW pre-treatment. This work highlights the practical feasibility of reclaimed water electrolysis, enabling wastewater facilities to contribute to the broader integration of clean energy transition.
