Affordable CO2 capture: How common clay could revolutionize the industry
A tablespoon of clay has about the same surface area as a football field; With a little tweaking, it can soak up as much CO2 as more expensive sorbents, researchers show.
June 12, 2025
Large-scale facilities that capture carbon dioxide from the air are already operating around the world. But the high-tech materials they use are expensive.
Researchers have now found that clay, one of the most abundant materials on Earth, could be a much simpler, cheaper way to for direct air capture of carbon dioxide. The results appear in The Journal of Physical Chemistry C.
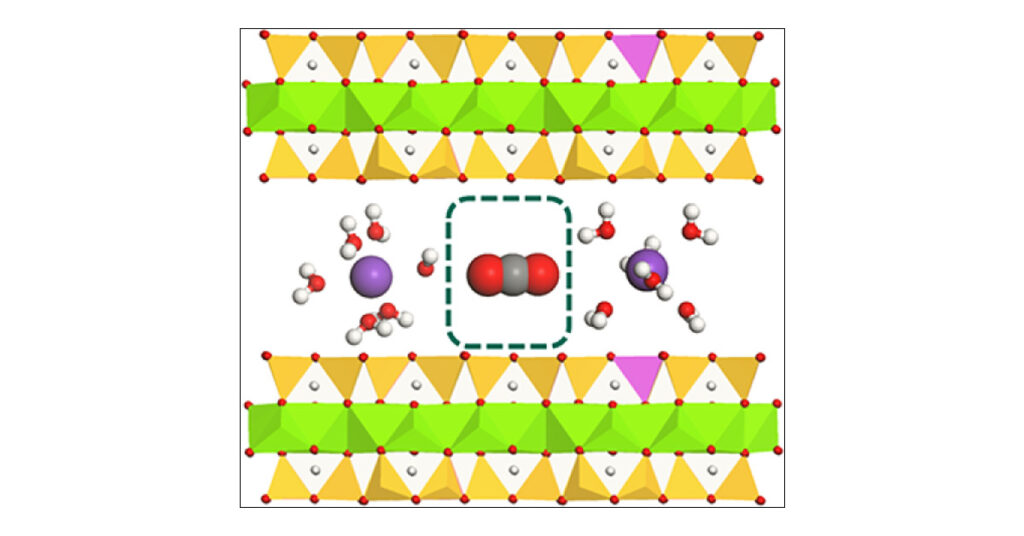
While clay minerals have been studied before to trap carbon dioxide, most of the work has focused on carbon sequestration in geological reservoirs where clay is present. In these scenarios, carbon dioxide is injected deep underground under high temperature and pressure. “Our work is novel because it showed that carbon dioxide can be sorbed on clays under ambient and near-ambient conditions,” said Cliff Johnston, professor of agronomy and earth sciences at Purdue in an email.
Many direct air capture systems today rely on advanced porous materials such as metal-organic frameworks and clay-like zeolites to capture carbon. Pores give these materials a high surface area to hold the carbon dioxide.
Pores give these materials a high surface area to hold the carbon dioxide.
Clay minerals such as bentonite and smectite also have a very high surface area: a tablespoon of clay has approximately the same surface area as an American football field. And these minerals are some of the most ubiquitous nanomaterials found on Earth.
a tablespoon of clay has approximately the same surface area as an American football field
Chemists have made the best carbon capture material yet
Johnston and colleagues have found in the past that these clays can bind significant quantities of highly toxic contaminants. This was surprising because these types of clays are generally water-loving, or hydrophilic, while the contaminants are exceptionally water-repelling, or hydrophobic, he says. “These results showed that clay interlayers have both hydrophilic and hydrophobic properties that can be manipulated,” he says.
This led to the team’s work on carbon dioxide capture on clays. They found that molecules like carbon dioxide are drawn to the hydrophobic regions of clay, while water vapor tends to occupy the hydrophilic regions.
“We have shown that these materials can sorb significant quantities of carbon dioxide, comparable to other direct capture sorbents, in clay interlayers,” Johnston says. “The surface chemistry and ability to bind carbon dioxide can be manipulated through geochemical controls and changes in humidity and temperature.”
He says that the team now has a patent application in process, and are trying to implement the technology on a large scale.
Source: Cliff T. Johnston et al. Competitive Sorption of H2O and CO2 in Clay Mineral Interlayers. J. Phys. Chem. C, 2025.
Abstract

The first-reported competitive CO2–H2O sorption isotherms on a naturally occurring expandable clay mineral (saponite) exchanged with Na, K, and Cs at near ambient CO2 concentrations (1% CO2) are reported by combining ATR-FTIR spectroscopy with gravimetric sorption methods. Little CO2 sorption was observed at high relative humidity (RH) but increased significantly at low RH. Unlike prior work where CO2 sorption was reported on samples that were previously subjected to heating and/or evacuation treatment to remove sorbed H2O, this study manipulated CO2 sorption by controlling RH. CO2sorption increased in the order Cs- > K- > Na-exchanged saponite and was anticorrelated with the Gibbs energy of hydration of the exchangeable cation. As expected, H2O sorption followed the opposite trend, with increased H2O sorption occurring in the order Na > K > Cs. The amount of CO2 sorbed using 1 atm CO2 (p/po = 1.0) ranged from 50 to 30 mgCO2/gclay and from 1.25 to 0.8 mgCO2/gclay for 1% CO2 (p/po = 0.01) in N2. The sensitivity of the ATR-FTIR measurements is demonstrated by detecting the CO2 stretch of adsorbed CO2 from air containing 500 ppmv CO2. The spectral features of sorbed CO2 were minimally affected by the nature of the exchangeable cation or variations in H2O content. However, the position of the HOH bending band shifted significantly from 1638 to 1610 cm–1 as H2O content decreased, reflecting changes in intermolecular hydrogen bonding between H2O molecules in the saponite interlayer. The shift in position of the HOH bending band to lower energy was coincident with increased CO2 sorption. CO2 sorption in clay mineral interlayers is consistent with sorption on weakly hydrated partially hydrophobic sites found on low charge density smectites and is enhanced by lower RH and by the presence of weakly hydrated exchangeable cations like Cs+ or K+. The greatest CO2 sorption occurs on Cs-saponite because this is the least hydrated cation of the three used in the study and indicates that CO2 sorption occurs on the neutral portion of the siloxane surface.
