Chemists turn plastic waste into carbon capture material
Researchers have developed a new chemical process that transforms discarded PET bottles into an efficient, durable carbon-capture sorbent—tackling both plastic pollution and greenhouse gas emissions at once.
September 18, 2025
Researchers have developed a new chemical process transforms plastic waste into an effective carbon capture material.
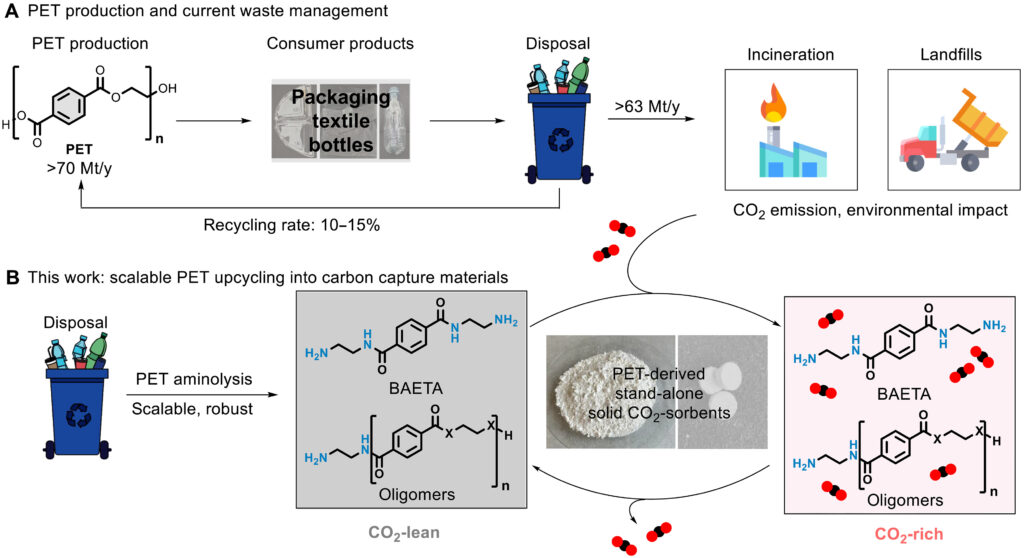
The method works with polyethylene terephthalate (PET), the ubiquitous clear plastic that beverage bottles and clothing fibers are made of. The plastic is recyclable, but it has little value so most of the discarded plastic ends up in landfills, incinerators, or as pollution in waterways and oceans.
The new method shows that “plastic waste can be upcycled into a high-value carbon-capture material, addressing two environmental challenges at once: plastic pollution and greenhouse-gas emissions,” says Ji-Woong Lee, a chemist at the University of Copenhagen and Aarhus University. Lee and his colleagues reported their work in the journal Science Advances.
To limit global warming to 1.5–2°C, experts believe that in addition to reducing the world’s carbon emissions, will also need to remove 1.5–2.6 billion metric tons of carbon dioxide from the atmosphere every year.
There are ways to remove carbon emissions from concentrated sources such as smokestacks or directly from the air. These carbon capture methods typically rely on liquids such as amines or solid sorbents, which are expensive or require a lot of heat to release the carbon dioxide they soak up.
Lee’s team has been working on amine-based sorbents for many years. “We realized that the building blocks we need for making stable sorbents could be derived directly from discarded PET bottles, potentially turning a liability into a climate solution,” he says.
Mix. Remove Nanoplastics. Repeat.
To make their new sorbent, the researchers treated PET waste at mild temperatures with a chemical called 1,2-ethylenediamine. After 24 hours, the reaction converted the PET into a powdery substance called bis-aminoamide (BAETA), which can be made into pellets for use in industrial exhaust pipes.
“Our synthesis uses relatively mild conditions and inexpensive reagents, and we have already demonstrated kilogram-scale batches using real PET waste,” Lee says. “While further engineering is needed, the chemistry itself is straightforward and compatible with existing polymer-processing infrastructure.”
BAETA captures carbon dioxide efficiently. Once it is saturated, BAETA releases carbon dioxide at a temperature of 150°C, lower than other amine-based sorbents. BAETA is also durable and does not degrade at temperatures of up to 250°C, making it suitable for high-heat exhaust systems. And the material works effectively for a long time. Under flue gas conditions, the its performance did not change after 40 cycles in 150 °C air.
This is not the first time researchers have made carbon capture materials from plastic waste. Three years ago, another team reported a way to heat plastic waste in the absence of oxygen to make an ultra-porous sponge-like material that soaks up carbon dioxide.
Lee and colleagues now plan to scale up and test the material under real-world conditions.
Source: Margarita Poderyte et al. Repurposing polyethylene terephthalate plastic waste to capture carbon dioxide. Sci. Adv. 2025.
