Researchers yoke the sun to distill ammonia fertilizer from wastewater
Making ammonia fertilizer is energy and carbon-intensive. New solar-driven method is a low-cost way to recycle the ammonia in farm and industrial runoff that would otherwise become pollution.
By Anthropocene Team August 7, 2025
Sewage is not something most people want to give a second thought to, but it contains a trove of valuable nutrients. One of those is ammonia, a key ingredient of fertilizer.
Now, researchers report a way to use sunlight to recover ammonia from wastewater. The cheap, efficient process is a practical way to reuse the nutrient on farms and keep it from reaching the environment, where it can cause harm. The work appears in the journal Nature Sustainability.
Ammonia is a source of nitrogen in fertilizers. Around 240 million tons of ammonia are produced every year globally using the Haber-Bosch process. The method, while critical for feeding the world, takes huge amounts of energy and has a large carbon footprint.
Meanwhile, two-thirds of the fertilizer farmers apply to their fields escapes into the ground as run-off. The excessive nitrogen reaches water bodies where it can harm aquatic life and lead to toxic algae blooms.
Designer bacteria clean wastewater and generate power—at the same time
Removing ammonia from wastewater is doable but the process is expensive so treatment plants mostly decompose ammonia into less harmful compounds. So researchers have been devising various techniques and developing novel materials to capture ammonia and other nutrients from wastewater.
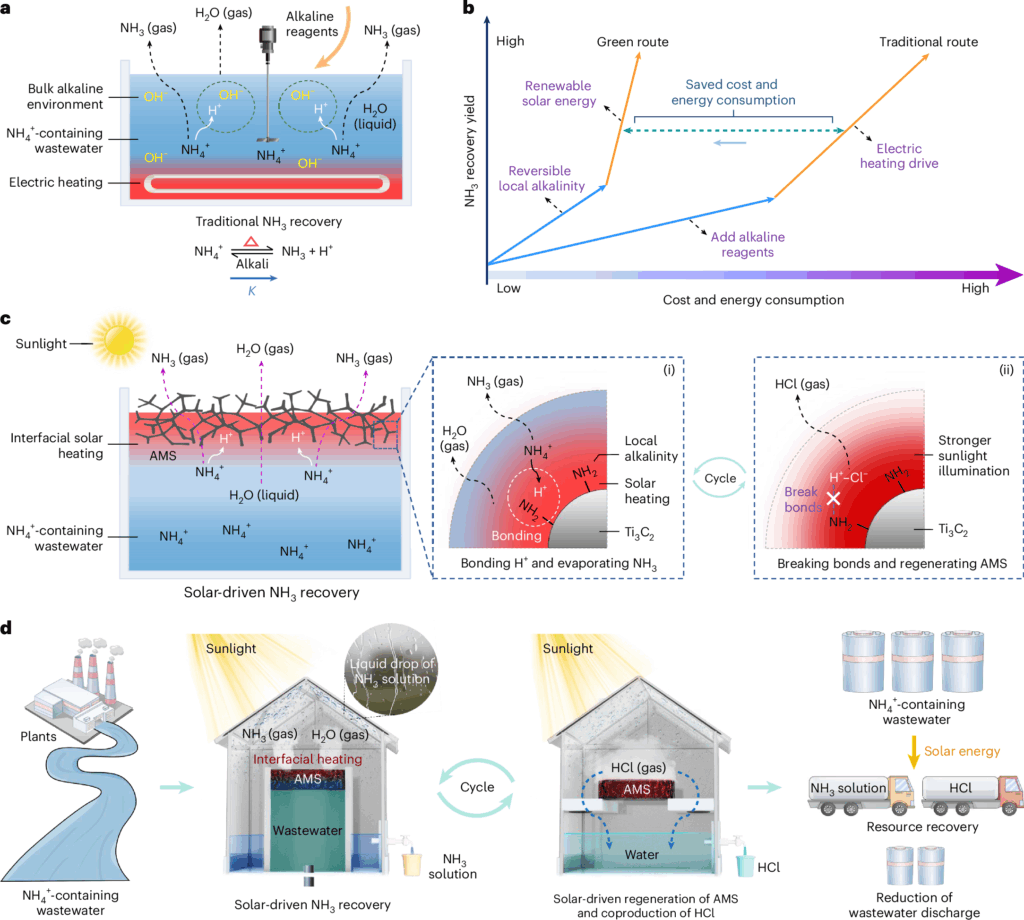
Ning Xu and colleagues at Nanjing University came up with an efficient solar-driven method. They made a solar still—a container with a clear plastic or glass top—that uses the sun to purify water. The sun’s heat evaporates water in the sill, and the clean vapors are condensed and collected.
Because fertilizer runoff and industrial wastewater mostly contain ammonia in the form of ammonium, the team devised a way to convert the ammonium into ammonia. They coated a plastic sponge with a thin layer of a black, heat-absorbing substance called titanium carbide. Then they attached chemical groups called amino groups to the surface of the sponge.
Floating on the wastewater enclosed in the solar still, the black sponge absorbed heat while its amino groups converted the ammonium in wastewater into ammonia. The heat evaporated both the ammonia and water, which were condensed and captured for use.
Focusing sunlight on the sponge cleans it for reuse while producing another useful commodity: hydrochloric acid. The researchers analyzed the economics of the method in 24 different regions of China. Taking into account the cost of the specialty sponge materials, they found that it had “excellent economics benefit and revenue” generating a profit of $90 per square meter of the sponge, and a payback period of about 3.5 years.
Source: Qi Zhang et al. Solar-driven efficient and selective ammonia recovery from ammonium-containing wastewater. Nature Sustainability, 2025.
References
- Service, R. F. New recipe produces ammonia from air, water, and sunlight. Science 345, 610–610 (2014).CAS Google Scholar
- Rosca, V., Duca, M., de Groot, M. T. & Koper, M. T. M. Nitrogen cycle electrocatalysis. Chem. Rev. 109, 2209–2244 (2009).CAS Google Scholar
- Wang, K. F. et al. Intentional corrosion-induced reconstruction of defective NiFe layered double hydroxide boosts electrocatalytic nitrate reduction to ammonia. Nat. Water 1, 1068–1078 (2023).CAS Google Scholar
- Comer, B. M. et al. Prospects and challenges for solar fertilizers. Joule 3, 1578–1605 (2019).CAS Google Scholar
- Li, Y. et al. Bipolar membrane electrodialysis for generation of hydrochloric acid and ammonia from simulated ammonium chloride wastewater. Water Res. 89, 201–209 (2016).CAS Google Scholar
- Chang, I. S. & Chung, C. M. Pollution prevention for manufacturing of ammonium chloride-an experimental study of wastewater recycling. Desalination 127, 145–153 (2000).CAS Google Scholar
- Li, J. Y. et al. Subnanometric alkaline-earth oxide clusters for sustainable nitrate to ammonia photosynthesis. Nat. Commun. 13, 1098 (2012).Google Scholar
- Huang, H., Xiao, X. & Yan, B. Complex treatment of the ammonium nitrogen wastewater from rare-earth separation plant. Desalin. Water Treat. 8, 109–117 (2012).Google Scholar
- Buelens, L. C., Galvita, V. V., Poelman, H., Detavernier, C. & Marin, G. B. Super-dry reforming of methane intensifies CO2 utilization via Le Chatelier’s principle. Science 354, 449–452 (2016).CAS Google Scholar
- Liu, B., Hu, F. & Shi, B. F. Recent advances on ester synthesis via transition-metal catalyzed C–H functionalization. ACS Catal. 5, 1863–1881 (2015).CAS Google Scholar
- Li, X. Q. et al. Graphene oxide-based efficient and scalable solar desalination under one sun with a confined 2D water path. Proc. Natl Acad. Sci. USA 113, 13953–13958 (2016).CAS Google Scholar
- Xu, N. et al. Going beyond efficiency for solar evaporation. Nat. Water 1, 494–501 (2023).Google Scholar
- Chen, C., Kuang, Y. & Hu, L. Challenges and opportunities for solar evaporation. Joule 3, 683–718 (2019).CAS Google Scholar
- Li, J. et al. Interfacial solar steam generation enables fast-responsive, energy-efficient, and low-cost off-grid sterilization. Adv. Mater. 30, 1805159 (2018).Google Scholar
- Li, Y. et al. 3D-printed, all-in-one evaporator for high-efficiency solar steam generation under 1 sun illumination. Adv. Mater. 29, 1700981 (2017).Google Scholar
- Zhou, L. et al. Quantifying hot carrier and thermal contributions in plasmonic photocatalysis. Science 362, 69–72 (2018).CAS Google Scholar
- Zhang, Q. et al. Vertically aligned Janus MXene-based aerogels for solar desalination with high efficiency and salt resistance. ACS Nano 13, 13196–13207 (2019).CAS Google Scholar
- He, S. et al. Nature-inspired salt resistant bimodal porous solar evaporator for efficient and stable water desalination. Energy Environ. Sci. 12, 1558–1567 (2019).CAS Google Scholar
- Zhao, F. et al. Highly efficient solar vapour generation via hierarchically nanostructured gels. Nat. Nanotechnol. 13, 486–495 (2018).Google Scholar
- Zielinski, M. S. et al. Hollow mesoporous plasmonic nanoshells for enhanced solar vapor generation. Nano Lett. 16, 2159–2167 (2016).CAS Google Scholar
- Li, X. Q. et al. Storage and recycling of interfacial solar steam enthalpy. Joule 2, 2477–2484 (2018).Google Scholar
- Wang, Z. X. et al. Versatile coating with multifunctional performance for solar steam generation. Nano Energy 74, 104886 (2020).CAS Google Scholar
- Ai, F. R. et al. Amino-functionalized Ti3C2 MXene quantum qots as photoluminescent sensors for diagnosing histidine in human serum. ACS Appl. Nano Mater. 4, 8192–8199 (2021).CAS Google Scholar
- Wu, Q. et al. Ultrasensitive and selective determination of carcinoembryonic antigen using multifunctional ultrathin amino-functionalized Ti3C2–MXene nanosheets. Anal. Chem. 92, 3354–3360 (2020).CAS Google Scholar
- Haddadi, S. A. et al. Amino-functionalized MXene nanosheets doped with Ce(III) as potent nanocontainers toward self-healing epoxy nanocomposite coating for corrosion protection of mild steel. ACS Appl. Mater. Interfaces 13, 42074–42093 (2021).CAS Google Scholar
- Zhang, G. L. et al. Synthesis of amino-functionalized Ti3C2Tx MXene by alkalization-grafting modification for efficient lead adsorption. Chem. Commun. 56, 11283–11286 (2020).CAS Google Scholar
- Yang, X. H. et al. Insights into the role of cation vacancy for significantly enhanced electrochemical nitrogen reduction. Appl. Catal. B 264, 118477 (2020).CAS Google Scholar
- Xu, N. et al. A water lily-inspired hierarchical design for stable and efficient solar evaporation of high-salinity brine. Sci. Adv. 5, eaaw7013 (2019).CAS Google Scholar
- Xu, W. C. et al. Flexible and salt resistant Janus absorbers by electrospinning for stable and efficient solar desalination. Adv. Energy Mater. 8, 1702884 (2018).Google Scholar
- Zhou, L. et al. 3D self-assembly of aluminium nanoparticles for plasmon-enhanced solar desalination. Nat. Photon. 10, 393–398 (2016).CAS Google Scholar
- Surwade, S. P. et al. Water desalination using nanoporous single-layer graphene. Nat. Nanotechnol. 10, 459–464 (2015).CAS Google Scholar
- Zhou, X. Y., Zhao, F., Guo, Y. H., Rosenberger, B. & Yu, G. H. Architecting highly hydratable polymer networks to tune the water state for solar water purification. Sci. Adv. 5, eaaw5484 (2019).CAS Google Scholar
- Ahmed, I., Khan, N. A., Yoon, J. W., Chang, J.-S. & Jhung, S. H. Protonated MIL-125-NH2: remarkable adsorbent for the removal of quinoline and indole from liquid fuel. ACS Appl. Mater. Interfaces 9, 20938–20946 (2017).CAS Google Scholar
- Rapti, S. A. et al. Selective capture of hexavalent chromium from an anion-exchange column of metal organic resin-alginic acid composite. Chem. Sci. 7, 2427–2436 (2016).CAS Google Scholar
- Luo, S. et al. Protonated NH2-MIL-125 via HCl vapor to introduce the moiety with charge and ample hydrogen as a novel bifunctional photocatalyst: enhanced photocatalytic H2 production and NO purification. Chem. Eng. J. 432, 134244 (2022).CAS Google Scholar
- Soloveichik, G. Electrochemical synthesis of ammonia as a potential alternative to the Haber–Bosch process. Nat. Catal. 2, 377–380 (2019).CAS Google Scholar
- Vinardell, S., Nicolas, P., Sastre, A. M., Cortina, J. L. & Valderrama, C. Sustainability assessment of green ammonia production to promote industrial decarbonization in Spain. ACS Sustain. Chem. Eng. 11, 15975–15983 (2023).CAS Google Scholar
- Yao, P. C. et al. Greener and higher conversion of esterification via interfacial photothermal catalysis. Nat. Sustain. 5, 348–356 (2022).Google Scholar
- Wankat, P. C. Separation Process Engineering (Pearson Education, 2006).
